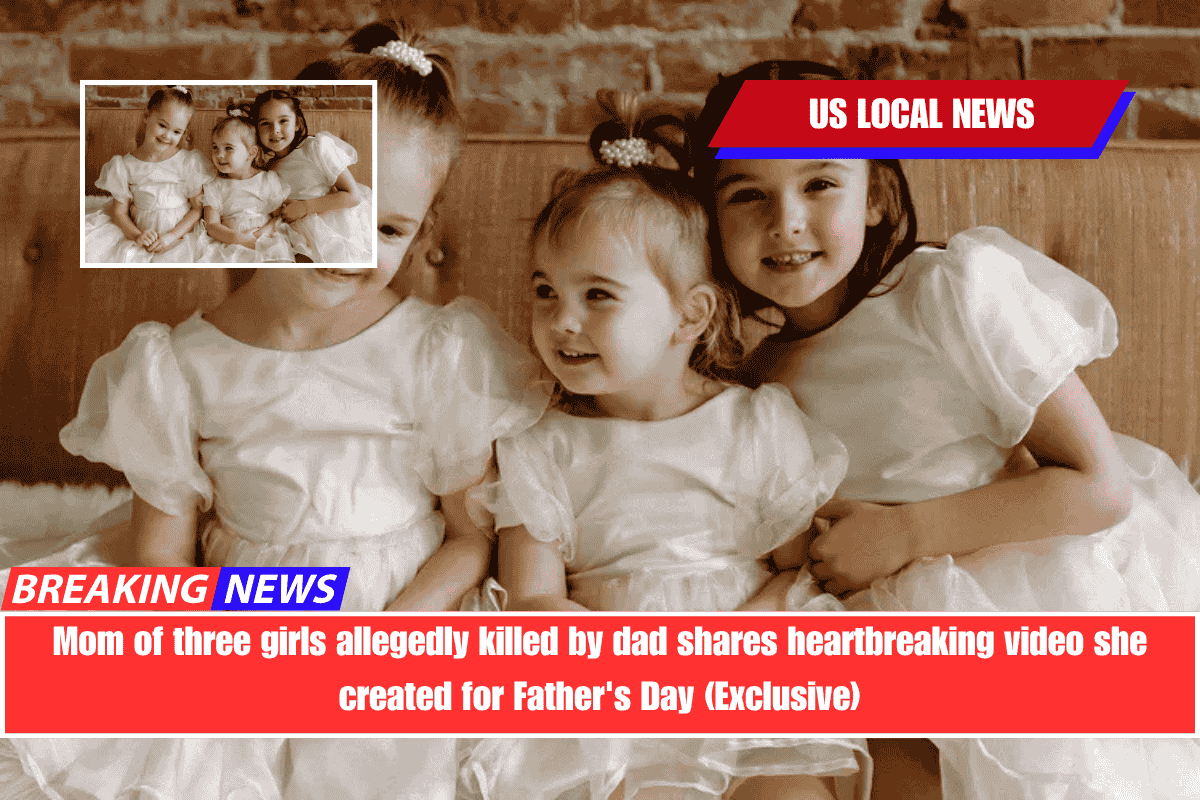
Before the tragic news broke that three girls had been discovered dead and their father was suspected of murdering them, their mother posted an online video tribute to her now-missing ex-husband, whom she claims was a good parent despite his mental health issues, according to her attorney.
The 20-second video, shared exclusively with PEOPLE on June 6 by Whitney Decker’s attorney Arianna Cozart, was created as a Father’s Day tribute to Travis Decker, Cozart says.
The video features a slideshow of Travis, Whitney, and their daughters, Paityn Decker, 9, Evelyn Decker, 8, and Olivia Decker, 5, as Pharrell Williams’ song “Happy” plays.
Whitney’s account is now private, so it’s unclear when the video with the TikTok logo was posted online.
Travis is suspected of murdering the girls, who were discovered dead on June 2 near the Rock Island Campground in Chelan County, Washington, where his pickup truck was also found. The sisters were reported missing on May 30 after failing to return on time from a “planned visitation” with Travis, according to police.
“Travis was a present and active father up until the end—still attending the girls’ soccer games, dance, and theater productions,” Cozart said in an emailed statement to PEOPLE on June 6. “Although Travis was struggling with his own mental health, he was a good co-parent, always communicating frequently with Whitney up until Friday evening when the girls went missing.”
According to Cozart, Decker and Whitney had been married for slightly more than seven years when “his mental health struggles, including his feelings of isolation, paranoia, and Borderline Personality Disorder, led to the crumbling of their marriage in 2022.” Travis, an Army veteran, sought mental health treatment but was unable to obtain it, according to the attorney.
Whitney later sought to limit his parenting time, as explained in a September 2024 parenting plan filed in Chelan County Superior Court and reviewed by PEOPLE.
In a separate filing, also seen by PEOPLE, she claimed Decker had “neglected his parental duties towards a child” and “has a long-term emotional or physical problem that gets in the way of his ability to parent.”
According to the parenting plan filing, he was granted three hours of visitation on Fridays and eight hours every other weekend as long as he remained in Wenatchee Valley. He was not granted overnight visits. The schedule was only temporary due to the court process, Cozart claims.
In the filing, Whitney also requested that Decker be evaluated for anger management/domestic violence, as well as a full psychiatric evaluation by a licensed psychiatrist that included any potential diagnoses. The filing stated that if Decker did not follow through on these things, the “mother will have the choice to further limit visitation.”
According to Cozart, Travis “was not a violent man with Whitney or the girls, ever.”
Travis is still on the run after being charged with three counts of first-degree murder and one count of kidnapping, as well as custodial interference.
The Chelan County Sheriff’s Office previously stated on Facebook that Decker is “well versed in wilderness survival and capable of spending days or even weeks in the wilderness on his own and with very little equipment.”
Authorities are requesting that anyone who sees Travis call 911 immediately and not approach him. People can also call the CCSO tip line at 509-667-6845 or send information to the line.